Steric control of the in/out sense of bridgehead substituents in macrobicyclic compounds: isolation of new “crossed chain” variants of in/out isomers†
Abstract
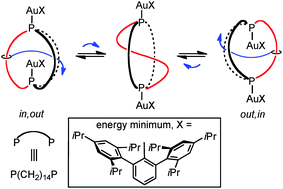
Isomers of the cage like dibridgehead diphosphine P((CH2)14)3P (1) are treated with Ph3PAu(2,6-C6H3(Trip)2) (2 equiv.; Trip = 2,4,6-C6H2(iPr)3). With out,out-1, workup gives out,out-1·(Au(2,6-C6H3(Trip)2))2 (46%), as confirmed by a crystal structure. With in,out-1, crystallization affords not in,out-1·(Au(2,6-C6H3(Trip)2))2, but rather an out,out isomer in which one of the (CH2)14 segments threads through the macrocycle formed by the other two. Implications for mechanisms of interconversion of in,out isomers are analyzed.

 Please wait while we load your content...
Please wait while we load your content...