Light-driven nitrile imine-mediated tetrazole–ene cycloaddition as a versatile platform for fullerene conjugation†
Abstract
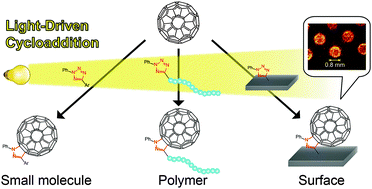
An efficient methodology for modular fullerene functionalization via the photo-induced nitrile imine-mediated tetrazole–ene cycloaddition (NITEC) is introduced. The versatility and platform character of the method is illustrated by the light-driven reaction of fullerenes with small molecule, polymeric and surface-immobilized tetrazoles. The efficient fullerene conjugation is evidenced via mass spectrometric techniques.

 Please wait while we load your content...
Please wait while we load your content...