Controlling intermolecular aurophilicity in emissive dinuclear Au(i) materials and their luminescent response to ammonia vapour†
Abstract
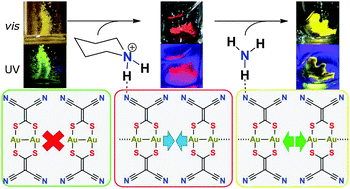
The concept that hydrogen bonding cations can reduce the coulombic repulsion inherent to anionic gold species and thereby trigger aurophilicity is realized with three new photoluminescent compounds of the form [Q]2[Au2(i-mnt)2] (i-mnt = (CN)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CS22−, Q = 3,5-dimethylpyrazolium, piperidinium). These compounds illustrate unprecedented supramolecular aurophilicity between the anions, the emission of which is significantly red-shifted compared to zero-dimensional analogues, a direct result of the aurophilic network. The piperidinium salt exhibits a vapochromic/luminescent response to ammonia, inducing a change in colour of the reflectance and emission from red to yellow. These results demonstrate the ability to rationally control the formation of supramolecular metallophilic networks via the incorporation of hydrogen bonding cations.
CS22−, Q = 3,5-dimethylpyrazolium, piperidinium). These compounds illustrate unprecedented supramolecular aurophilicity between the anions, the emission of which is significantly red-shifted compared to zero-dimensional analogues, a direct result of the aurophilic network. The piperidinium salt exhibits a vapochromic/luminescent response to ammonia, inducing a change in colour of the reflectance and emission from red to yellow. These results demonstrate the ability to rationally control the formation of supramolecular metallophilic networks via the incorporation of hydrogen bonding cations.

 Please wait while we load your content...
Please wait while we load your content...