A novel visible light mediated radical cyclization of enol lactones: a concise method for fluorinated polycyclic lactone scaffolds†
Abstract
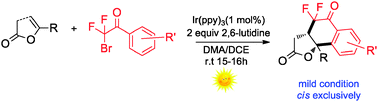
A novel visible light mediated radical cyclization of enol lactones with difluoroacyl arenes is presented. The reaction experienced a tandem radical cyclization and tolerated a wide range of substrates, resulting in fluorinated γ-butyrolactones in good chemical yield and with excellent diastereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...