Rhodium-catalysed alkoxylation/acetalization of diazo compounds: one-step synthesis of highly functionalised quaternary carbon centres†
Abstract
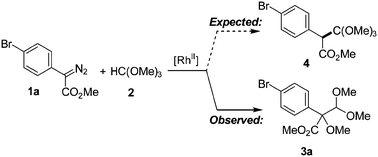
An intermolecular tandem reaction for the rapid build-up of densely functionalised α-alkoxy-β-oxo-esters has been developed. This novel process applies the easy to handle trimethyl orthoformate as a C1-building block in the rhodium(II)-catalysed alkoxylation/acetalization of donor–acceptor substituted diazo compounds. The concomitant C–O/C–C bond formation reaction gives products with unique quaternary carbon centers, substituted by groups of different oxidation level (ester, protected aldehyde and alkoxide).

 Please wait while we load your content...
Please wait while we load your content...