KOtBu-mediated annulation of acetonitrile with aldehyde: synthesis of substituted dihydropyridin-2(1H)-ones, pyridin-2(1H)-ones, and thiopyridin-2(1H)-ones†
Abstract
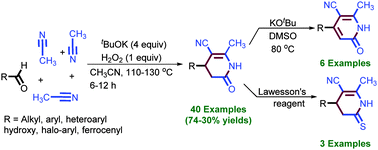
The KOtBu-mediated annulation of acetonitrile with aldehyde was observed, in which the cleavage of four C(sp3)–H bonds occurred and a total of eight new bonds were formed during the synthesis of substituted dihydropyridinones in the presence of peroxide. Furthermore, dihydropyridinones have been transformed into pyridinones using KOtBu in DMSO.


 Please wait while we load your content...
Please wait while we load your content...