Unprecedented χ isomers of single-side triol-functionalized Anderson polyoxometalates and their proton-controlled isomer transformation†
Abstract
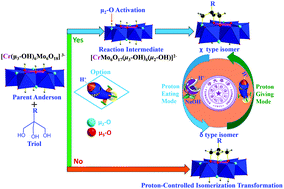
The μ2-O atom in Anderson polyoxometalates was regioselectively activated by the introduction of protons, which, upon functionalization with triol ligands, could afford a series of unique χ isomers of the organically-derived Anderson cluster {[RCC(CH2O)3]MMo6O18(OH)3}3−. Herein proton-controlled isomer transformation between the δ and χ isomer was observed by using the fingerprint region in the IR spectra and 13C NMR spectra.

 Please wait while we load your content...
Please wait while we load your content...