Thioamides in the collagen triple helix†
Abstract
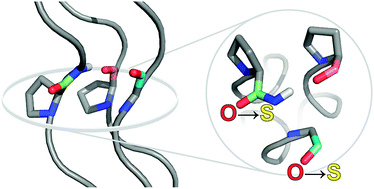
To probe noncovalent interactions within the collagen triple helix, backbone amides were replaced with a thioamide isostere. This subtle substitution is the first in the collagen backbone that does not compromise thermostability. A triple helix with a thioamide as a hydrogen bond donor was found to be more stable than triple helices assembled from isomeric thiopeptides.

 Please wait while we load your content...
Please wait while we load your content...