sp2–sp3 diboranes: astounding structural variability and mild sources of nucleophilic boron for organic synthesis
Abstract
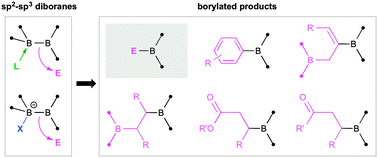
Despite the widespread use of organoborane reagents in organic synthesis and catalysis, a major challenge still remains: very few boron-centered nucleophiles exist for the direct construction of B–C bonds. Perhaps the most promising emerging solution to this problem is the use of sp2–sp3 diboranes, in which one boron atom of a conventional diborane(4) is quaternised by either a neutral or anionic nucleophile. These compounds, either isolated or generated in situ, serve as relatively mild and convenient sources of the boryl anion [BR2]− for use in organic synthesis and have already proven their efficacy in metal-free as well as metal-catalysed borylation reactions. This Feature article documents the history of sp2–sp3 diborane synthesis, their properties and surprising structural variability, and their burgeoning utility in organic synthesis.

 Please wait while we load your content...
Please wait while we load your content...