A novel 1,8-naphthalimide derivative with an open space for an anion: unique fluorescence behaviour depending on the binding anion's electrophilic properties†
Abstract
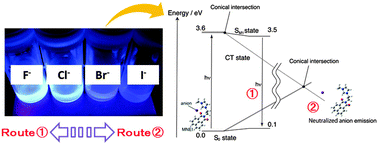
We have designed a novel 1,8-naphthalimide derivative with an open space for an anion. Computational calculation has predicted that the space could trap various anion species and photo-induced charge transfer depending on the anion's electrophilic properties. Indeed, the fluorescence behaviour of the 1,8-naphthalimide derivative complexes with each anion is consistent with the computational prediction.

 Please wait while we load your content...
Please wait while we load your content...