Catalyst-controlled reverse selectivity in C–C bond formation: NHC-Cu-catalyzed α-selective allylic alkylation with organolithium reagents†
Abstract
An efficient and highly α-selective copper-catalyzed allylic alkylation of allylic halides with organolithium reagents is presented. The use of N-heterocyclic carbenes as ligands is key to reverse the common γ-selectivity of this transformation and gives rise to the corresponding linear products with high levels of regioselectivity.
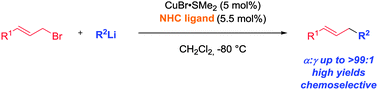

 Please wait while we load your content...
Please wait while we load your content...