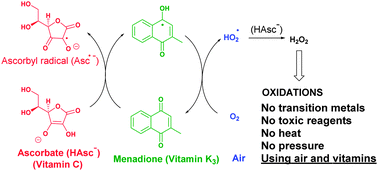
Abstract
Transition metal-free oxidation with air at room temperature has been achieved by simply using ascorbate (vitamin C) and catalytic amounts of menadione (vitamin K3). A combination of the mentioned vitamins transforms atmospheric oxygen into hydrogen peroxide, which is able to oxidize arylboronic acids and other chemical moieties.

 Please wait while we load your content...
Please wait while we load your content...