Catalysis at the room temperature ionic liquid|water interface: H2O2 generation†
Abstract
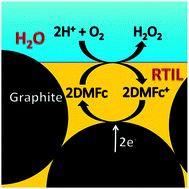
H2O2 is produced at the interface between a room-temperature ionic liquid with decamethylferrocene as an electron donor and an acidic aqueous solution. The electron donor can be regenerated electrochemically.

 Please wait while we load your content...
Please wait while we load your content...