Spectroscopic determination of relative Brønsted acidity as a predictor of reactivity in aprotic ionic liquids†
Abstract
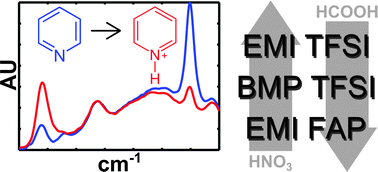
A simple titration technique utilizing pyridine as a FTIR spectroscopy probe is demonstrated to successfully predict relative Brønsted acid-limited reaction rates in different ionic liquid (IL) environments. Relative acidity is shown to vary across three aprotic ILs in a manner that is specific to the particular acid–IL pairing.

 Please wait while we load your content...
Please wait while we load your content...