Asymmetric Suzuki–Miyaura cross-coupling of 1-bromo-2-naphthoates using the helically chiral polymer ligand PQXphos†
Abstract
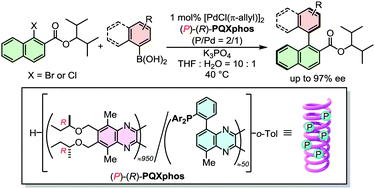
A single-handed helical polymer ligand PQXphos afforded axially chiral biaryl esters with high enantioselectivities in asymmetric Suzuki–Miyaura cross-coupling. The use of naphthyl bromide bearing a 2,4-dimethyl-3-pentyl ester resulted in both high yields and high enantioselectivities. Either enantiomer could be synthesized selectively by using a single PQXphos through a solvent-dependent switch of the helical chirality.

 Please wait while we load your content...
Please wait while we load your content...