Abstract
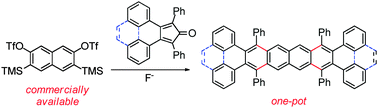
Phenyl-substituted tetra-, penta-, hexa- and octacenes were easily obtained starting from a readily available naphthalene-based bisaryne precursor. This approach to large acenes involves a sequence of two Diels–Alder cycloadditions with dienones followed by two CO extrusion reactions.
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...