Palladium-catalysed carbonylative α-arylation of nitromethane†
Abstract
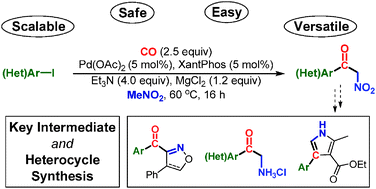
A simple and mild Pd-catalysed carbonylative α-arylation of nitromethane has been realised providing access to α-nitro aryl ketones from an array of aryl and heteroaryl iodides. The methodology requires only a mild base and uses the convenient solid CO releasing molecule, COgen in a two-chamber system. Changing to the isotopically labelled 13COgen, [13C]-acyl labelling can be effected through the generation of a near stoichiometric amount of 13CO. Lastly, the significance of the generated products as synthetic intermediates is demonstrated.

 Please wait while we load your content...
Please wait while we load your content...