Protein stabilization by an amphiphilic short monodisperse oligo(ethylene glycol)†
Abstract
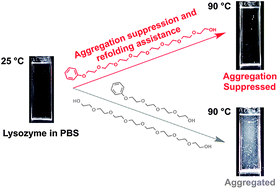
A short, monodisperse additive (octa(ethylene glycol) monophenyl ether) functions to suppress aggregation of thermally and chemically denatured lysozyme. Control studies with shorter and non-amphiphilic derivatives revealed that the amphiphilic structure is essential, and octa(ethylene glycol) is nearly the minimum chain length for amphiphilic poly(ethylene glycol)s to stabilize proteins.

 Please wait while we load your content...
Please wait while we load your content...