Lipase active site covalent anchoring of Rh(NHC) catalysts: towards chemoselective artificial metalloenzymes†
Abstract
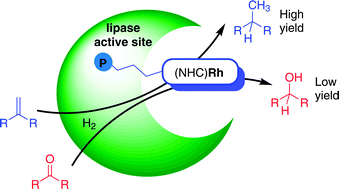
A Rh(NHC) phosphonate complex reacts with the lipases cutinase and Candida antarctica lipase B resulting in the first (soluble) artificial metalloenzymes formed by covalent active site-directed hybridization. When compared to unsupported complexes, these new robust hybrids show enhanced chemoselectivity in the (competitive) hydrogenation of olefins over ketones.

 Please wait while we load your content...
Please wait while we load your content...