Stacking interactions by two Phe side chains stabilize and orient assemblies of even the minimal amphiphilic β-sheet motif†
Abstract
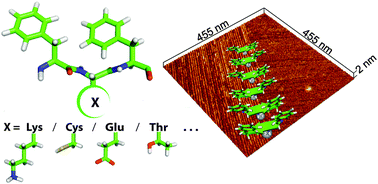
Here we demonstrate that the smallest possible motif of the amphiphilic and pleated β-strand structure can be generated using tri-peptides stabilized by π–π stacking interactions. Monitoring the early stages of Phe-Glu-Phe fibril formation revealed unique angular orientations. Phe-Glu-Phe fibrils were further exploited as adsorbing templates for metal ions.

 Please wait while we load your content...
Please wait while we load your content...