Iron catalysed cross-couplings of azetidines – application to the formal synthesis of a pharmacologically active molecule†
Abstract
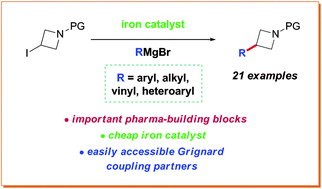
A protocol for the coupling of 3-iodoazetidines with Grignard reagents in the presence of an iron catalyst has been developed. A variety of aryl, heteroaryl, vinyl and alkyl Grignards were shown to participate in the coupling process to give the products in good to excellent yields. Furthermore, a short formal synthesis towards a pharmacologically active molecule was shown.

 Please wait while we load your content...
Please wait while we load your content...