C–H activation dependent Pd-catalyzed carbonylative coupling of (hetero)aryl bromides and polyfluoroarenes†‡
Abstract
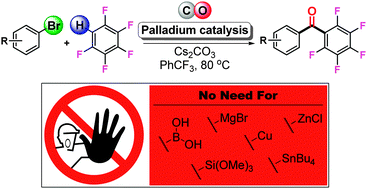
The carbonylative coupling of aryl and heteroaryl bromides with polyfluoroarenes via palladium-catalyzed C–H activation is presented. This transformation proceeds efficiently at moderate reaction temperatures and does not require strong base or reactive intermediates. A near stoichiometric amount of CO is sufficient and the methodology can thus be easily expanded to include the preparation of [13C]-acyl labeled benzopolyfluorophenones.

 Please wait while we load your content...
Please wait while we load your content...