Facile synthesis of borofragments and their evaluation in activity-based protein profiling†
Abstract
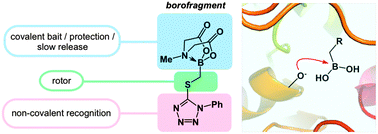
The discovery of enzyme inhibitors relies on synthetic methods that enable rapid and modular construction of small molecules. Heterocyclic fragments designed to maximize enthalpic interactions with their protein targets represent a particularly desirable class of molecules. Here we describe a reagent that enables straightforward construction of “borofragments”, in which a heterocycle is separated from the boron center by two or three rotatable bonds. The stability of these molecules depends on the MIDA group which likely acts as a slow-release element under biological conditions. Borofragments can be used to discover inhibitors of enzymes that use catalytic oxygen nucleophiles. We have employed this method to identify inhibitors of ABHD10 and the predicted carboxypeptidase CPVL. This technique should be applicable to other classes of targets.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners

 Please wait while we load your content...
Please wait while we load your content...