A facile and selective route to remarkably inert monocyclic NHC-stabilized boriranes†
Abstract
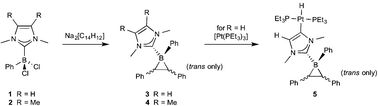
Herein we report a facile and selective synthetic route to monocyclic NHC-stabilized boriranes. We have succeeded in obtaining two highly stable new boriranes through salt elimination of NHC-stabilized dichloroboranes with the dianion of trans-stilbene, Na2[C14H12]. One borirane was observed to undergo reaction with [Pt(PEt3)3], in which the Pt(0) center oxidatively adds a backbone C–H bond of the NHC, leading to the isolation of the Pt(II) complex trans-[(Et3P)2PtH{C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(NMe)2C·BPh(C14H12)}]. The remarkable inertness of the NHC-boriranes suggests a strong stabilising effect of quaternization of the boron atom.
CH(NMe)2C·BPh(C14H12)}]. The remarkable inertness of the NHC-boriranes suggests a strong stabilising effect of quaternization of the boron atom.

 Please wait while we load your content...
Please wait while we load your content...