Influence of the chirality of short peptide supramolecular hydrogels in protein crystallogenesis†
Abstract
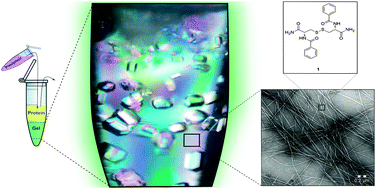
For the first time the influence of the chirality of the gel fibers in protein crystallogenesis has been studied. Enantiomeric hydrogels 1 and 2 were tested with model proteins lysozyme and glucose isomerase and a formamidase extracted from B. cereus. Crystallization behaviour and crystal quality of these proteins in both hydrogels are presented and compared.


 Please wait while we load your content...
Please wait while we load your content...