A novel one-pot method for the synthesis of substituted furopyridines: iodine-mediated oxidation of enaminones by tandem metal-free cyclization†
Abstract
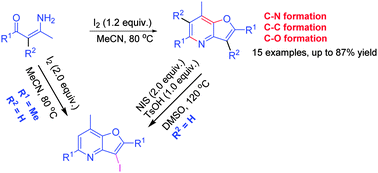
A novel iodine-mediated oxidative tandem cyclization reaction of simple enaminones has been developed for the synthesis of substituted furopyridines through C–C/C–N/C–O bond formation in a one-pot procedure. Substituted furopyridines are obtained in moderate to good yield. In addition, I- and Br-substituted furopyridines have been successfully produced by the electrophilic substitution of N-iodo- or N-bromosuccinimide.

 Please wait while we load your content...
Please wait while we load your content...