Diastereoselective synthesis of propargylic N-hydroxylamines via NHC–copper(i) halide-catalyzed reaction of terminal alkynes with chiral nitrones on water†
Abstract
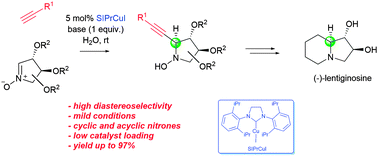
The first NHC–copper(I)-halide catalyzed addition of terminal alkynes to enantiomerically pure nitrones on water is described. This reaction provides a straightforward access to propargylic N-hydroxylamines in excellent yield and with excellent stereoselectivity (up to 97%). The presented methodology was applied as a key step in the formal synthesis of (−)-lentiginosine.

 Please wait while we load your content...
Please wait while we load your content...