Protecting group free enantiospecific total syntheses of structurally diverse natural products of the tetrahydrocannabinoid family†
Abstract
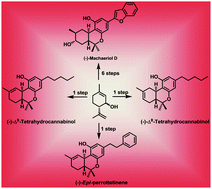
A simple, highly diastereoselective, Lewis acid catalyzed Friedel–Crafts coupling of a cyclic allylic alcohol with resorcinol derivatives has been developed. The method was applied for the enantiospecific total syntheses of structurally diverse natural products such as machaeriol-D, Δ8-THC, Δ9-THC, epi-perrottetinene and their analogues. Synthesis of both natural products and their enantiomers has been achieved with high atom economy, in a protecting group free manner and in less than 6 steps, the longest linear sequence, in a very good overall yield starting from R-(+) and S-(−)-limonene.

 Please wait while we load your content...
Please wait while we load your content...