First-principles study of electronic structure and photocatalytic properties of MnNiO3 as an alkaline oxygen-evolution photocatalyst†
Abstract
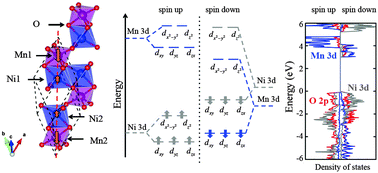
We present a first-principles study of MnNiO3, a promising oxygen-evolution photocatalyst. Using density functional theory with the PBE + U functional and the screened hybrid functional of Heyd, Scuseria, and Ernzerhof (HSE), we compute and analyze the ground-state geometry and electronic structure. We find that MnNiO3 is a ferrimagnetic semiconductor with an indirect band gap, consistent with experimental observations. We also predict that MnNiO3 has promising band edge positions relative to the vacuum, with potential to straddle the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) redox potentials in aqueous solution. A detailed analysis of the band structure and density of states provides a clear explanation for why MnNiO3 has appropriate electronic properties for OER. Furthermore, comprehensive calculations of its Pourbaix diagram suggest that MnNiO3 is stable in alkaline solution at potentials relevant for oxygen evolution.


 Please wait while we load your content...
Please wait while we load your content...