Catalytic, highly enantioselective, direct amination of enecarbamates†
Abstract
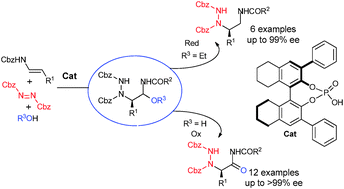
Amination of enecarbamates with dibenzylazodicarboxylate and oxygenated nucleophiles in the presence of a catalytic amount of chiral phosphoric acid afforded optically active stable precursors of α-hydrazinoimines, which were reduced or oxidized, respectively, to vicinal diamines or α-amino acid precursors with excellent yield and enantioselectivity.
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...