The direct α-C(sp3)–H functionalisation of N-aryl tetrahydroisoquinolines via an iron-catalysed aerobic nitro-Mannich reaction and continuous flow processing†‡
Abstract
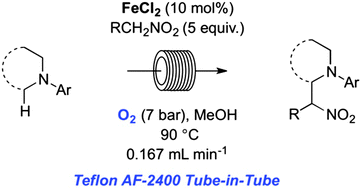
An efficient nitro-Mannich type direct α-C(sp3)–H functionalisation of N-aryl-1,2,3,4-tetrahydroisoquinolines catalysed by simple iron salts in combination with O2 as the terminal oxidant is described. The use of a Teflon AF-2400 membrane Tube-in-Tube reactor under continuous flow conditions allowed for considerable process intensification to be achieved relative to previous batch methods.
- This article is part of the themed collection: Recent Advances in Flow Synthesis and Continuous Processing

 Please wait while we load your content...
Please wait while we load your content...