Catalytic hydrogen production from paraformaldehyde and water using an organoiridium complex†
Abstract
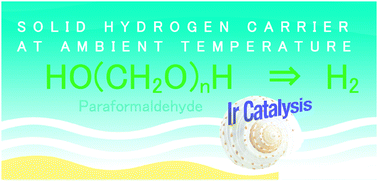
Paraformaldehyde was decomposed using an organoiridium complex (1, [IrIII(Cp*)(4-(1H-pyrazol-1-yl-κN2)benzoic acid-κC3)(H2O)]2SO4) as a catalyst in water to produce H2 and CO2 in a 2 : 1 molar ratio at room temperature. The catalytic cycle is composed of the reduction of 1 by paraformaldehyde under basic conditions to produce formic acid and the hydride complex, which reacts with protons to produce H2. Formic acid further decomposed to H2 and CO2 with 1.

 Please wait while we load your content...
Please wait while we load your content...