The interplay of fibronectin functionalization and TGF-β1 presence on fibroblast proliferation, differentiation and migration in 3D matrices†
Abstract
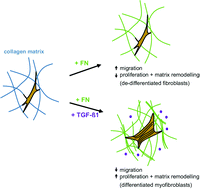
Defined biomimetic three-dimensional (3D) matrices are needed to decipher the complex cellular signalling during wound healing at high resolution in vitro. Soluble factors like TGF-β1 and adhesion promoting structural components of the extracellular matrix (ECM) are known to be key regulators of fibroblast behaviour. The ECM component fibronectin (FN) bears a complex function as adhesion promoter, fibrillar element and soluble factor binder. However, its implementation in biomimetic 3D matrices is frequently ill defined. To study the impact of FN on fibroblast cellular function under differentiating conditions (TGF-β1 stimulation), we functionalized 3D collagen I matrices with FN using two strategies: co-assembly and adsorptive immobilization. In comparison to co-assembly, adsorptive immobilization provided no alteration in collagen microstructure as well as mechanical properties. Moreover, this approach provided a controllable FN amount and a homogenous distribution of FN throughout collagen networks. A strong interplay of FN amount and TGF-β1 stimulation on fibroblast function was found in terms of proliferation, migration and myofibroblast differentiation. High levels of FN alone reduced proliferation and showed no effect on differentiation of fibroblasts, but increased migration. In contrast, fibroblast stimulation with high amounts of FN together with TGF-β1 increased proliferation. Independent of FN, the TGF-β1 stimulation enhanced mRNA expression of matrix components like collagen type I alpha 1 chain (Coll I(a1), FN with extra domain A (EDA-FN) and reduced cell migration. The latter cell behaviour indicated a FN independent differentiation into a myofibroblast phenotype. Overall, our 3D biomimetic matrices allow dissecting the overlapping action of the ECM protein FN and the soluble factor TGF-β1 on fibroblast proliferation, migration and differentiation in 3D microenvironments. Furthermore, this model enables the mimicking of important steps of the in vivo wound healing process in vitro.


 Please wait while we load your content...
Please wait while we load your content...