Engineering personalized neural tissue by combining induced pluripotent stem cells with fibrin scaffolds
Abstract
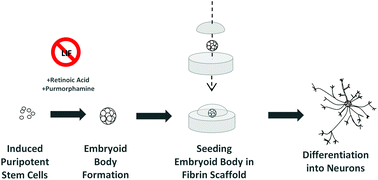
Induced pluripotent stem cells (iPSCs) are generated from adult somatic cells through the induction of key transcription factors that restore the ability to become any cell type found in the body. These cells are of interest for tissue engineering due to their potential for developing patient-specific therapies. As the technology for generating iPSCs advances, it is important to concurrently investigate protocols for the efficient differentiation of these cells to desired downstream phenotypes in combination with biomaterial scaffolds as a way of engineering neural tissue. For such applications, the generation of neurons within three dimensional fibrin scaffolds has been well characterized as a cell-delivery platform for murine embryonic stem cells (ESCs) but has not yet been applied to murine iPSCs. Given that iPSCs have been reported to differentiate less effectively than ESCs, a key objective of this investigation is to maximize the proportion of iPSC-derived neurons in fibrin through the choice of differentiation protocol. To this end, this study compares two EB-mediated protocols for generating neurons from murine iPSCs and ESCs: an 8 day 4−/4+ protocol using soluble retinoic acid in the last 4 days and a 6 day 2−/4+ protocol using soluble retinoic acid and the small molecule sonic hedgehog agonist purmorphamine in the last 4 days. EBs were then seeded in fibrin scaffolds for 14 days to allow further differentiation into neurons. EBs generated by the 2−/4+ protocol yielded a higher percentage of neurons compared to those from the 4−/4+ protocol for both iPSCs and ESCs. The results demonstrate the successful translation of the fibrin-based cell-delivery platform for use with murine iPSCs and furthermore that the proportion of neurons generated from murine iPSC-derived EBs seeded in fibrin can be maximized using the 2−/4+ differentiation protocol. Together, these findings validate the further exploration of 3D fibrin-based scaffolds as a method of delivering neuronal cells derived from iPSCs – an important step toward the development of iPSC-based tissue engineering strategies for spinal cord injury repair.

 Please wait while we load your content...
Please wait while we load your content...