A colorimetric approach for measuring mercuric ions with high selectivity using label-free gold nanoparticles and thiourea†
Abstract
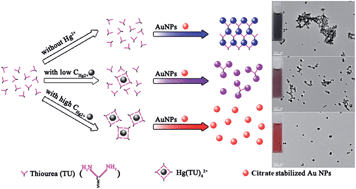
We report a simple colorimetric assay for Hg2+ measurements using label-free gold nanoparticles (Au NPs, 13.0 ± 1.6 nm) with the aid of thiourea (TU). The addition of TU into Au NPs resulted in aggregation of Au NPs with a red-to-blue color change. While in the presence of Hg2+, it inhibited the aggregation of Au NPs induced by TU, resulting in a reverse color change from blue to purple and finally to wine red. These color variations are dependent on the Hg2+ concentration and can be utilized for the colorimetric sensing of Hg2+. This assay requires only 3 min to reach a naked-eye detection limit of 100 nM, which satisfies the guideline concentration of Hg2+ (250 nM) in industrial water regulated by the EPA. And the concentration of Hg2+ is quantitatively determined using a UV-vis spectrophotometer with a limit of detection (LOD) of 40 nM. The response of the assay caused by Hg2+ (2 μM) was 6.0 to 9.1 times higher than those induced by other metal ions (100 μM), indicating its high specificity towards Hg2+. The assay has been applied for the analysis of Hg2+ in drinking and lake water samples with recoveries in the range of 98.3–120.0%.

 Please wait while we load your content...
Please wait while we load your content...