A comparative study for recognizing G-quadruplexes using dimeric cyanine dyes with different sizes of aromatic substituents†
Abstract
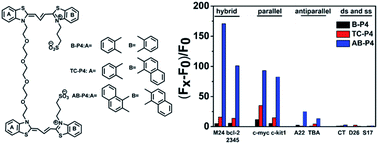
A series of dimeric cyanine dyes with different sizes of heterocyclic aromatic substituents and the same linker were synthesized, and these dyes were demonstrated to be highly selective compounds for binding to G-quadruplex DNA. These results showed that the size of heterocyclic aromatic substituents had great influence on the self-assembly properties of these dyes as well as their interaction with G-quadruplex motifs. A dye with a large aromatic substituent (AB-P4) formed strong aggregates and also presented significant selectivity for the G-quadruplex over duplex DNA. In contrast, dyes with small aromatic rings (B-P4 and TC-P4) had a much smaller effect on the recognition of the G-quadruplex, suggesting that the possession of a suitably sized plane for good π–π stacking with the G-quadruplex was essential for the interaction of dyes with the G-quadruplex. Moreover, the loop structures of the G-quadruplex also affected the binding mode of AB-P4 with the G-quadruplex: loops located at the side of the tetrad were favorable for both end-stacking binding modes in the form of monomers, while more hindrance by the loop at one end of the tetrads resulted in one end-stacking on the G-quadruplex in the form of dimers. In comparison, loops play weak roles in dyes with TC-P4, and this dye could stack on both ends of tetrads.

 Please wait while we load your content...
Please wait while we load your content...