Site-selective characterization of Src homology 3 domain molecular recognition with cyanophenylalanine infrared probes†
Abstract
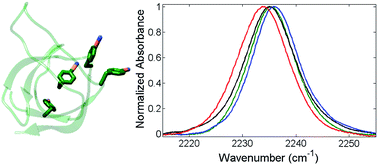
Local heterogeneity of microenvironments in proteins is important in biological function, but difficult to characterize experimentally. One approach is the combination of infrared (IR) spectroscopy and site-selective incorporation of probe moieties with spectrally resolved IR absorptions that enable characterization within inherently congested protein IR spectra. We employed this method to study molecular recognition of a Src homology 3 (SH3) domain from the yeast protein Sho1 for a peptide containing the proline-rich recognition sequence of its physiological binding partner Pbs2. Nitrile IR probes were introduced at four distinct sites in the protein by selective incorporation of p-cyanophenylalanine via the amber codon suppressor method and characterized by IR spectroscopy. Variation among the IR absorption bands reports on heterogeneity in local residue environments dictated by the protein structure, as well as on residue-dependent changes upon peptide binding. The study informs on the molecular recognition of SH3Sho1 and illustrates the speed and simplicity of this approach for characterization of select microenvironments within proteins.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...