Colorimetric detection of bisphenol A using Au–Fe alloy nanoparticle aggregation†
Abstract
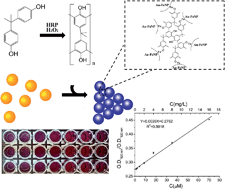
A colorimetric assay for bisphenol A (BPA) detection was developed based on Au–Fe alloy nanoparticles. Au–Fe alloy nanoparticles were synthesized and optimized by a hydrothermal method. In the colorimetric assay, horseradish peroxidase was introduced to catalyze BPA polymerization in the presence of hydrogen peroxide, which greatly enhances the sensitivity of the assay. Selectivity is achieved by specific phenol–Fe3+ interactions between BPA and the Au–Fe alloy nanoparticles. The limit of detection of BPA is 0.58 μM (S/D = 3). The ratio of absorption at 620 nm to absorption at 520 nm is linear with the BPA concentration in the range of 1.10 μM (0.25 mg L−1) to 70.1 μM (16 mg L−1). Parallel spiked and real polycarbonate samples were tested using the proposed method, and the obtained recoveries ranged from 95% to 103% for different samples. This level of detection is on par or slightly better than that achieved by HPLC using UV detection, which is routinely employed to detect BPA. Compared with HPLC, the colorimetric assay for BPA detection exhibits higher throughput and lower cost, making it suitable for the extensive screening of BPA in samples.

 Please wait while we load your content...
Please wait while we load your content...