Structural dependency of collagen fibers on ion types revealed by in situ second harmonic generation (SHG) imaging method†
Abstract
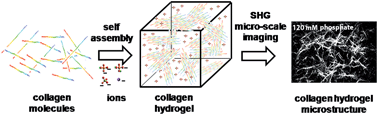
Ionic species in aqueous solutions alter protein solubility and aggregation behavior through a variety of complex interactions. Employing second harmonic generation (SHG) imaging in a backscattering configuration in situ we discovered that added phosphate modulates the aggregated collagen fibers' lengths within 3D hydrogels. For example, the about 1 μm in length collagen fibers formed in 30 mM phosphate-only buffer, 37 °C, 2 g l−1 collagen solid content extended to about 45 μm and increased in width in high (>/ = 60 mM) phosphate. Adding sodium sulfate in a 30 mM phosphate buffer to polymerize collagen into a hydrogel at 37 °C had similar effects. On the other hand, adding sodium chloride did not lengthen collagen fibers. The fiber lengths and widths decreased in very high concentrations of all salts. To establish the timescales of the involved polymerization processes, we used traditional turbidity measurements of gelation. Based on the solubility experiments we concluded that over 85% of collagen had precipitated under all experimental conditions. The non-invasive in situ SHG imaging in this study is valuable because it reduces the possibility of artifacts associated with changes to the fragile collagen hydrogels taking place in the conventional electron and optical imaging experiments.

 Please wait while we load your content...
Please wait while we load your content...