Analysis of ofloxacin and flavoxate HCl either individually or in combination via a green chromatographic approach with a pharmacokinetic study of ofloxacin in biological samples
Abstract
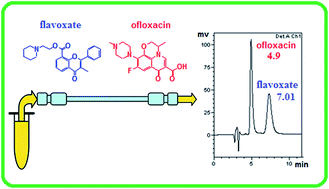
A new sensitive and rapid micellar liquid chromatographic method was developed for the determination of ofloxacin (OFL) and flavoxate hydrochloride (FLV) in different pharmaceutical formulations. The analyses were carried out on a BDS Hypersil phenyl column (4.6 mm × 250 mm, 5 μm particle size) using a micellar mobile phase consisting of 0.15 M sodium dodecyl sulfate, 15% n-propanol, 0.3% triethylamine, and 0.02 M orthophosphoric acid (pH 2.5) with UV detection at 325 nm. The proposed method exhibited good linearity over the concentration range of 2.0–40.0 μg mL−1 for the two drugs with lower detection limits of 0.16 μg mL−1 for OFL and 0.32 μg mL−1 for FLV. The proposed method was applied to the determination of OFL and FLV in their co-formulated and single ingredient dosage forms with good percentage recoveries (99.86–99.98%) and small standard deviation values (±0.18 to ±1.26). The results of the proposed method were statistically compared with those obtained by the comparison methods revealing no significant differences in the performance of the two methods regarding accuracy and precision. To achieve high sensitivity that allows the pharmacokinetic study of OFL, fluorescence detection at 290/485 nm was employed. A linear relationship was achieved for OFL in plasma and urine over the concentration ranges of 0.01–0.10 and 0.01–0.20 μg mL−1 with mean percentage recoveries of 100.8 ± 2.85% and 101.1 ± 2.78%, respectively. A pharmacokinetic study of OFL in human plasma and urine was conducted.

 Please wait while we load your content...
Please wait while we load your content...