Effects of charge states, charge sites and side chain interactions on conformational preferences of a series of model peptide ions†
Abstract
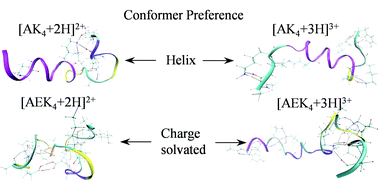
The effects of charge states, charge sites and side chain interactions on conformational preferences of gas-phase peptide ions are examined by ion mobility-mass spectrometry (IM-MS) and molecular dynamics (MD) simulations. Collision cross sections (CCS) of [M + 2H]2+ and [M + 3H]3+ ions for a series of model peptides, viz. Ac-(AAKAA)nY-NH2 (AKn, n = 3–5) and Ac-Y(AEAAKA)nF-NH2 (AEKn, n = 2–5) are measured by using IM-MS and compared with calculated CCS for candidate ions generated by MD simulations. The results show that charge states, charge sites and intramolecular charge solvation are important determinants of conformer preference for AKn and AEKn ions. For AKn ions, there is a strong preference for helical conformations near the N-terminus and charge-solvated conformations near the C-terminus. For [AEKn + 2H]2+ ions, conformer preferences appear to be driven by charge solvation, whereas [AEKn + 3H]3+ ions favor more extended coil-type conformations.
- This article is part of the themed collection: Ion Mobility Mass Spectrometry

 Please wait while we load your content...
Please wait while we load your content...