Aqueous-filled polymer microcavity arrays: versatile & stable lipid bilayer platforms offering high lateral mobility to incorporated membrane proteins†
Abstract
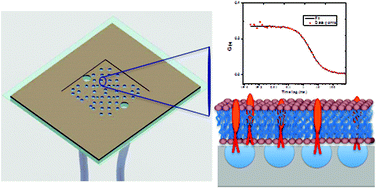
A key prerequisite in an ideal supported lipid bilayer based cell membrane model is that the mobility of both the lipid matrix and its components are unhindered by the underlying support. This is not trivial and with the exception of liposomes, many of even the most advanced approaches, although accomplishing lipid mobility, fail to achieve complete mobility of incorporated membrane proteins. This is addressed in a novel platform comprising lipid bilayers assembled over buffer-filled, arrays of spherical cap microcavities formed from microsphere template polydimethoxysilane. Prior to bilayer assembly the PDMS is rendered hydrophilic by plasma treatment and the lipid bilayer prepared using Langmuir Blodgett assembly followed by liposome/proteoliposome fusion. Fluorescence Lifetime Correlation Spectroscopy confirmed the pore suspended lipid bilayer exhibits diffusion coefficients comparable to free-standing vesicles in solution. The bilayer modified arrays are highly reproducible and stable over days. As the bilayers are suspended over deep aqueous reservoirs, reconstituted membrane proteins experience an aqueous interface at both membrane interfaces and attain full lateral mobility. Their utility as membrane protein platforms was exemplified in two case studies with proteins of different dimensions in their extracellular and cytoplasmic domains reconstituted into DOPC lipid bilayers; Glycophorin A, and Integrin αIIbβ3. In both cases, the proteins exhibited 100% mobility with high lateral diffusion coefficients.

 Please wait while we load your content...
Please wait while we load your content...