Quantitative analysis of sitagliptin using the 19F-NMR method: a universal technique for fluorinated compound detection
Abstract
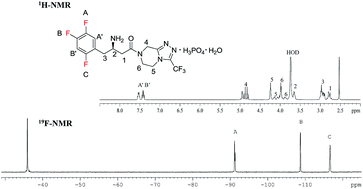
To expand the application scope of nuclear magnetic resonance (NMR) technology in quantitative analysis of pharmaceutical ingredients, 19F nuclear magnetic resonance (19F-NMR) spectroscopy has been employed as a simple, rapid, and reproducible approach for the detection of a fluorine-containing model drug, sitagliptin phosphate monohydrate (STG). ciprofloxacin (Cipro) has been used as the internal standard (IS). Influential factors, including the relaxation delay time (d1) and pulse angle, impacting the accuracy and precision of spectral data are systematically optimized. Method validation has been carried out in terms of precision and intermediate precision, linearity, limit of detection (LOD) and limit of quantification (LOQ), robustness, and stability. To validate the reliability and feasibility of the 19F-NMR technology in quantitative analysis of pharmaceutical analytes, the assay result has been compared with that of 1H-NMR. The statistical F-test and student t-test at 95% confidence level indicate that there is no significant difference between these two methods. Due to the advantages of 19F-NMR, such as higher resolution and suitability for biological samples, it can be used as a universal technology for the quantitative analysis of other fluorine-containing pharmaceuticals and analytes.

 Please wait while we load your content...
Please wait while we load your content...