In situ high-energy synchrotron X-ray diffraction revealing precipitation reaction kinetics of silver ions with mixed halide ions†
Abstract
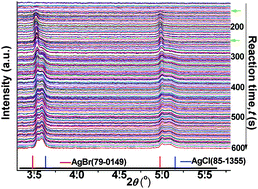
Precipitation of silver ions simultaneously with chloride and bromide ions in ethylene glycol at a mild temperature (e.g., 60 °C) has been successfully demonstrated for the synthesis of silver chlorobromide (AgClxBr1−x, 0 < x < 1) nanoparticles, which is realized by injecting a AgNO3 solution into a solution containing both halogen ions. The injection rate of the AgNO3 solution has been determined to be critical for controlling the uniformity of AgClxBr1−x nanoparticles. Time-resolved in situ high-energy synchrotron X-ray diffraction has been applied, for the first time, to quantitatively monitor the reaction kinetics of nanocrystal formation. The real-time results shed light on the fact that the injection rate of AgNO3 solution significantly influences the nucleation and growth processes, and thus the quality of resulting AgClxBr1−x nanoparticles. Specifically, fast injection enables the complete addition of AgNO3 solution to the reaction solution before the nucleation process starts, leading to a good separation of nucleation and growth and thus the formation of uniform AgClxBr1−x nanocubes with well-defined composition and narrow size distribution. By contrast, slow injection results in a continuous addition of AgNO3 solution to the reaction solution even after nucleation starts, leading to continuous multiple nucleation/growth processes and thus the formation of AgClxBr1−x nanoparticles with broad dimensional and morphological distributions.

 Please wait while we load your content...
Please wait while we load your content...