Temperature dependent structural, elastic, and polar properties of ferroelectric polyvinylidene fluoride (PVDF) and trifluoroethylene (TrFE) copolymers
Abstract
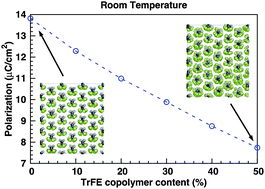
We use molecular dynamics to calculate the structural, elastic, and polar properties of crystalline ferroelectric β-poly(vinylidene fluoride), PVDF (–CH2–CF2–)n with randomized trifluoroethylene TrFE (–CHF–CF2–)n as a function of TrFE content (0–50%) in the temperature range of 0–400 K. There is a very good agreement between the experimentally obtained and the computed values of the lattice parameters, thermal expansion coefficients, elastic constants, polarization, and pyroelectric coefficients. A continuous decrease in Young's modulus with increasing TrFE content was observed and attributed to the increased intramolecular and intermolecular repulsive interactions between fluorine atoms. The computed polarization displayed a similar trend, with the room temperature spontaneous polarization decreasing by 44% from 13.8 μC cm−2 (pure PVDF) to 7.7 μC cm−2 [50/50 poly(VDF-co-TrFE)]. Our results show that molecular dynamics can be used as a practical tool to predict the mechanical and polarization-related behavior of ferroelectric poly(VDF-co-TrFE). Such an atomistic model can thus serve as a guide for practical applications of this important multifunctional polymer.

 Please wait while we load your content...
Please wait while we load your content...