Ambipolar charge-transport properties in 4,10-dihalogenated anthanthrone crystals: a theoretical study†
Abstract
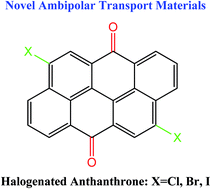
The charge-transport parameters in three 4,10-dihalogenated anthanthrones (AAOs) are investigated by means of density functional theory (DFT) and molecular dynamics (MD) calculations. Our calculations point to similar hole and electron reorganization energies for each molecule. Significant electronic couplings and bandwidths (particularly for electron transport) are found along the parallel π–π stacking directions in all the dihalogenated AAO crystals. The calculated effective masses are small or moderate for both holes and electrons. Especially for the iodinated AAO crystals, remarkable ambipolar charge transport can be anticipated due to the smallest and similar effective masses for holes and electrons (both are around 1.0 m0). In addition, due to the presence of two small effective masses, two-dimensional charge transport would take place for electrons in the chlorinated AAO crystal and for both holes and electrons in the iodinated AAO crystal. Also, our calculations reveal large nonlocal electron–phonon couplings along the π-stacks in the brominated AAO and, in particular, the chlorinated AAO crystals, which can further improve the balance in transport of holes and electrons.

 Please wait while we load your content...
Please wait while we load your content...