Anion exchange membranes composed of perfluoroalkylene chains and ammonium-functionalized oligophenylenes†
Abstract
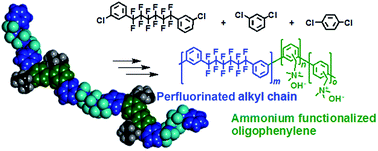
A novel series of ammonium-containing copolymers (QPAFs) were synthesized as anion exchange membranes for alkaline fuel cell applications. The precursor copolymers (Mw = 28.3–90.1 kDa) composed of perfluoroalkylene and phenylene groups were obtained by a nickel promoted polycondensation reaction. Chloromethylation and quaternization reactions of the precursors provided thin and ductile QPAF membranes with ion exchange capacity (IEC) ranging from 0.79 to 1.74 meq g−1. The QPAF membranes exhibited a phase-separated morphology based on the hydrophilic/hydrophobic differences in the main chain structure. The QPAF membrane with an optimized copolymer composition and IEC = 1.26 meq g−1 showed high hydroxide ion conductivity (95.5 mS cm−1 in water at 80 °C), excellent mechanical properties (large elongation at break (218%)), and reasonable alkaline stability at 80 °C. An alkaline fuel cell using the QPAF as the membrane and electrode binder achieved the maximum power density of 139 mW cm−2 at a current density of 420 mA cm−2.


 Please wait while we load your content...
Please wait while we load your content...