Improving the energy density of Na3V2(PO4)3-based positive electrodes through V/Al substitution†
Abstract
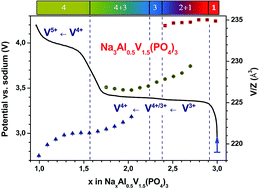
The crystal chemistry and the electrochemical properties upon Na+ extraction/insertion of NASICON-type Na3AlyV2−y(PO4)3 compositions (y = 0.1, 0.25 and 0.5) were investigated. It was found that this family of V/Al substituted NASICON materials undergoes multiple reversible phase transitions between −50 °C and 250 °C upon heating, from monoclinic to rhombohedral symmetry, related to progressive disordering of Na+ ions within the framework. Na+ insertion/extraction mechanisms were monitored by operando X-ray diffraction. It is shown for the first time that substitution of aluminum for vanadium in Na3Al0.5V1.5(PO4)3 increases significantly the theoretical energy density of these promising positive electrodes (425 W h kg−1) due to its lighter molecular weight and the possibility of reversible operation on the V4+/V5+ redox couple at 3.95 V vs. Na+/Na.

 Please wait while we load your content...
Please wait while we load your content...