Nanostructured terbium-doped ceria spheres: effect of dopants on their physical and chemical properties under reducing and oxidizing conditions†
Abstract
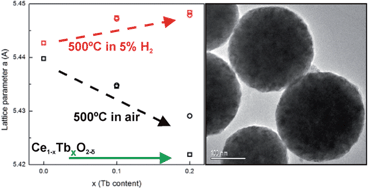
In this work, nanostructured Ce1−xTbxO2−δ (x = 0.1 and 0.2) spheres were synthesized by microwave assisted hydrothermal homogeneous co-precipitation and their properties were characterized by synchrotron radiation X-ray diffraction (SR-XRD), X-ray absorption spectroscopy (XAS) and scanning and high resolution electron microscopy (SEM and HRTEM) with energy dispersive X-ray spectroscopy (EDS). Spherical particles with average diameters around 200 nm were obtained in excellent yields. In order to compare the effect of the morphology on the physico-chemical properties, terbium-doped ceria nanopowders were also synthesized by a cation complexation method. In situ SR-XRD, X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) experiments were carried out under reducing and oxidizing conditions in order to investigate the redox behaviour of these materials and to evaluate the oxidation state ratios in the Ce3+/Ce4+ and Tb3+/Tb4+ couples. All of the Ce1−xTbxO2−δ samples were found to have a cubic crystal structure (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group). The spheres were composed of nanoparticles with an average crystallite size of about 10 nm. In situ XRD experiments showed an increase in lattice parameters on reduction which was attributed to the reduction of Ce4+ and Tb4+ cations to Ce3+ and Tb3+, which have larger radii, and to the associated increase in the VO concentration. The effect of the synthesis method on structural properties was evident in that the percentage of Tb present as Tb3+ in the nanostructured spheres was larger than that in the nanopowders of the same elemental composition.
m space group). The spheres were composed of nanoparticles with an average crystallite size of about 10 nm. In situ XRD experiments showed an increase in lattice parameters on reduction which was attributed to the reduction of Ce4+ and Tb4+ cations to Ce3+ and Tb3+, which have larger radii, and to the associated increase in the VO concentration. The effect of the synthesis method on structural properties was evident in that the percentage of Tb present as Tb3+ in the nanostructured spheres was larger than that in the nanopowders of the same elemental composition.

 Please wait while we load your content...
Please wait while we load your content...