Capsular polypyrrole hollow nanofibers: an efficient recyclable adsorbent for hexavalent chromium removal†
Abstract
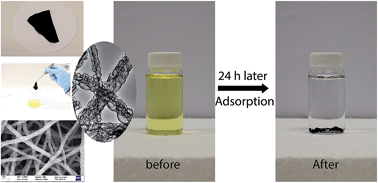
Capsular polypyrrole hollow nanofibers (PPy-HNFs) were fabricated via in situ polymerization of pyrrole on an organic–inorganic template, followed by acid etching. Their application in removing hexavalent chromium (Cr(VI)) from aqueous solution was then investigated. The morphologies of the capsular PPy-HNFs were studied by both scanning electron microscopy (SEM) and transmission electron microscopy (TEM), which showed that the PPy-HNFs had a capsular structure in the walls of hollow nanofibers. Fourier transform infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS) data confirmed the adsorption of Cr on capsular PPy-HNFs. The adsorption capacity increased with reduced pH of the initial solution and the adsorption process can be described using the pseudo-second-order model. These capsular PPy-HNFs showed a high Cr(VI) adsorption capacity up to 839.3 mg g−1. This adsorption capacity was largely retained even after five adsorption/desorption cycles. Electrostatic attraction between Cr and PPy-HNFs was studied using a proposed adsorption mechanism. The capsular PPy-HNFs formed a flexible membrane, which allowed easy handling during application. This study has demonstrated the possibilities of using this capsular PPy-HNF membrane for heavy metal removal from aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...